U.S., August 16,2020
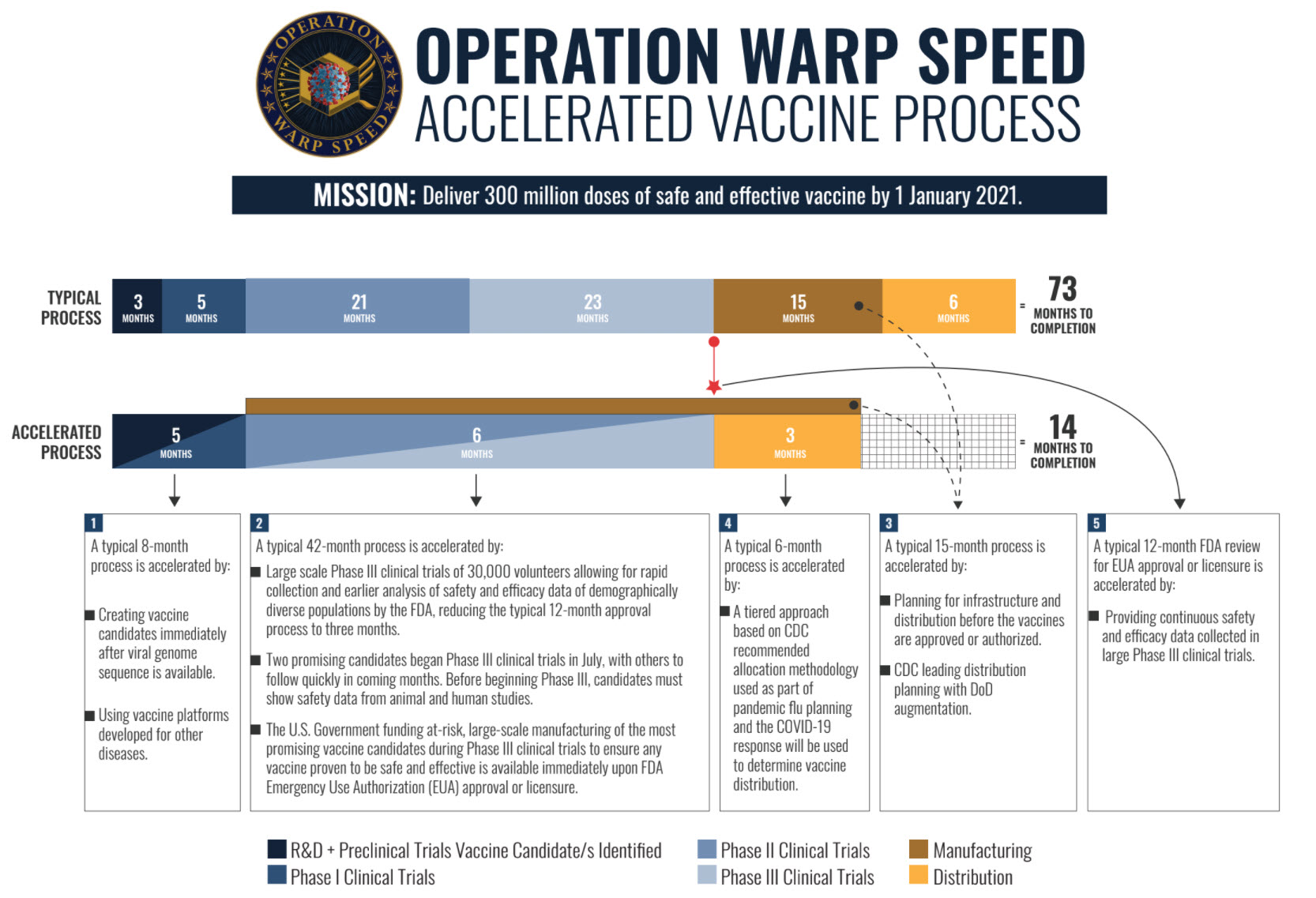
On May 15, 2020 the White House announced Operation Warp Speed (OWS) to
accelerate development, production, and distribution of China Plague (COVID-19)
vaccines, therapeutics and diagnostics in order to produce 300 million safe and effective
vaccine doses available for all Americans by January 1, 2021.
Few have taken time to observe the remarkable structure created to make this happen.
Structure is necessary to serve people by executing and achieving such epic goals, and
against the backdrop of anarchists here and there feverishly ripping and tearing at
America’s structure, the operative and logistical structure of OWS is being delivered
largely by America’s military structure which the anarchists disdain. An interesting
social studies observation.
OWS is cochaired by Defense Secretary Dr. Mark T Esper and Health and Human
Services Secretary Alex Azar, with Dr. Moncef Slaoui designated as chief advisor and
Gen. Gustave F. Perna confirmed to serve as chief operating officer.
Contributing stakeholders are Department of Defense, Department of Health and
Human Services, including the Centers for Disease Control and Prevention, and US
Food and Drug Administration, the National Institutes of Health and the Biomedical
Advanced Research and Development Authority
OWS has five focus areas: development and testing of vaccines; development and
testing of therapeutics; development and testing of diagnostics; supply and production
of vaccines, diagnostics and therapeutics; security and assurance of vaccine, diagnostic
and therapeutic development, testing, supply, production and distribution.
OWS is ensuring 100% of all medical protocols are executed to standard.
OWS is swiftly working to deliver solutions through rapid investment in the most
promising medical counter measures.
OWS is leveraging the most talented experts from across the federal government and
private industry.
Using the resources of the federal government and the U.S. private sector, OWS is
accelerating the testing, supply, development, and distribution of safe and effective
vaccines, therapeutics, and diagnostics to counter the China Plague (COVID-19) by
January 2021.
The OWS timeline of achievements as of August 16, 2020 is:
August 14, 2020. CDC selects McKesson Corporation to support distribution of COVID-
19 vaccines and related supplies.
August 13, 2020. Congressional/media engagement on OWS vaccine prioritization.
August 11, 2020. $1.5 billion agreement with Moderna to support the large-scale
manufacture and delivery of a vaccine candidate.August 5, 2020. $1 billion agreement with Johnson & Johnson (Janssen) to support the
large-scale manufacturing and delivery of a vaccine candidate.August 4, 2020. $160 million awarded to Grand River Aseptic Manufacturing, Inc. for
domestic aseptic fill and finish manufacturing capacity for critical vaccines and
therapeutics.
July 31, 2020. $2 billion agreement with Sanofi and GlaxoSmithKline to support the
advanced development, including clinical trials and large-scale manufacturing, of a
vaccine candidate.
July 30, 2020. Congressional/media engagement on OWS vaccines.
July 27, 2020. Moderna and Pfizer begin Phase 3 clinical trials. Texas A&M University
and FUJIFILM announce a task order with Texas A&M University and FUJIFILM to
advance domestic manufacturing capabilities and capacity for a potential COVID-19
vaccine.
July 22, 2020. $1.95 billion agreement with Pfizer for the large-scale manufacturing and
nationwide distribution of 100 million doses of their vaccine candidate.
July 13, 2020. Congressional/media engagement on OWS vaccines.
July 7, 2020. $1.6 billion agreement to support the large-scale manufacturing of
Novavax’s vaccine candidate.
July 2, 2020. GEN Gustave Perna is Senate-confirmed to serve as chief operating
officer.
June 18, 2020. GEN Gustave Perna confirmation hearing.
June 15, 2020. Congressional/media engagement on OWS vaccines.
June 11, 2020. $143 million agreement with SiO2 Materials Science to ramp up
capacity to produce the company’s glass-coated plastic container.
June 9, 2020. $204 million agreement with Corning to expand the domestic
manufacturing capacity to produce approximately 164 million Valor Glass vials per year.
June 1, 2020. Emergent BioSolutions announces a task order to advance domestic
manufacturing capabilities and capacity for a potential COVID-19 vaccine as well as
therapeutics.
May 21, 2020. $1.2 billion agreement in support for AstraZeneca’s candidate vaccine.
May 15, 2020. White House announces Operation Warp Speed, or OWS.
May 12, 2020. $138 million contract with ApiJect for more than 100 million prefilled
syringes for distribution across the United States by year-end.
April 16, 2020. $483 million agreement in support available for Moderna’s candidate
vaccine, which began Phase 1 trials on March 16, 2020, and received a fast-track
designation from the U.S. Food & Drug Administration.
March 30, 2020. $456 million for Johnson & Johnson’s candidate vaccine.